
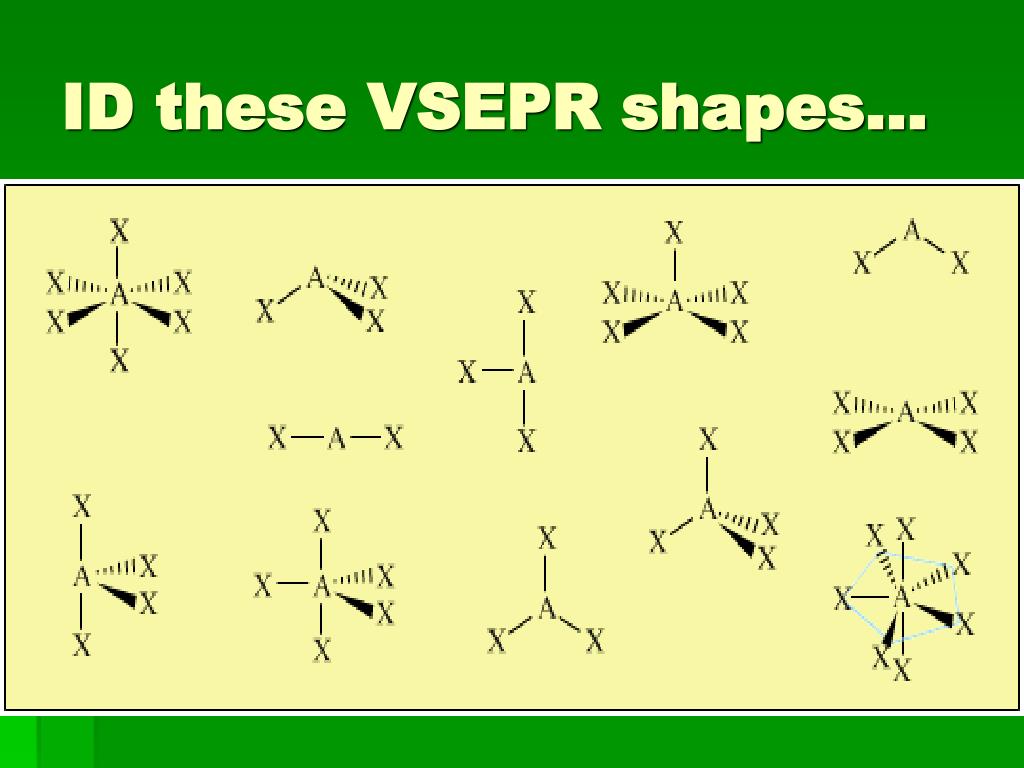
Unlike the trigonal planar, the trigonal bipyramidal structure is three-dimensional. Trigonal Bipyramidal: The molecule’s shape resembles a pyramid with a triangular base. This shape is because the four bond pairs experience minimum repulsion when the bonds are directed toward the corners of the tetrahedron. Tetrahedral: This shape occurs when one atom occupies the center, and four others are located at the corners of a tetrahedron. Examples are boron trifluoride (BF 3), boron trichloride (BCl 3), and sulfur trioxide (SO 3).ģ. It has one atom at the center and three at the corners of an equilateral triangle, making a bond angle of 120°. Trigonal Planar: The molecule forms a triangular shape in one plane. Examples of molecules with linear geometry are carbon dioxide (CO 2), beryllium chloride (BeCl 2), and nitric oxide (NO).Ģ. The atoms are arranged in a straight line, and the angle between the bonds is 180 °. Linear: It refers to the geometry shaped by a central atom surrounded by two other atoms. The VSEPR theory describes five fundamental shapes of molecules. The molecule’s energy will be at its lowest, thereby increasing stability. Since the electron pairs repel each other, they will remain at a maximum distance.For resonance structures, the VSEPR theory is individually applied to each structure.As a result, the shape is distorted, and the bond angles are reduced. When lone pairs are introduced into the basic structure, they squeeze the bond pairs closer together. A molecule consisting of only bond pairs forms the basic structure.Lone pair-lone pair > lone pair-bond pair > bond pair-bond pair We observe the following order of strength: The strength of repulsion is stronger among lone pairs than among bond pairs.In this way, they achieve the lowest energy and the most stable arrangement. The electron pairs tend to arrange themselves to minimize the repulsion and maximize the space between them.

They are classified into domains of electron-dense regions consisting of single bonds, double bonds, triple bonds, and lone pairs. These electron pairs include both bonding and nonbonding pairs. The total number of valence shell electron pairs determines the molecular shape.A polyatomic molecule consists of three or more atoms. The central atom in polyatomic molecules is surrounded by several other atoms.Therefore, it is also known as Gillespie-Nyholm theory. The VSEPR theory was developed by British chemist Ronald Gillespie and Australian chemist Ronald Nyholm and subsequently published in 1957.
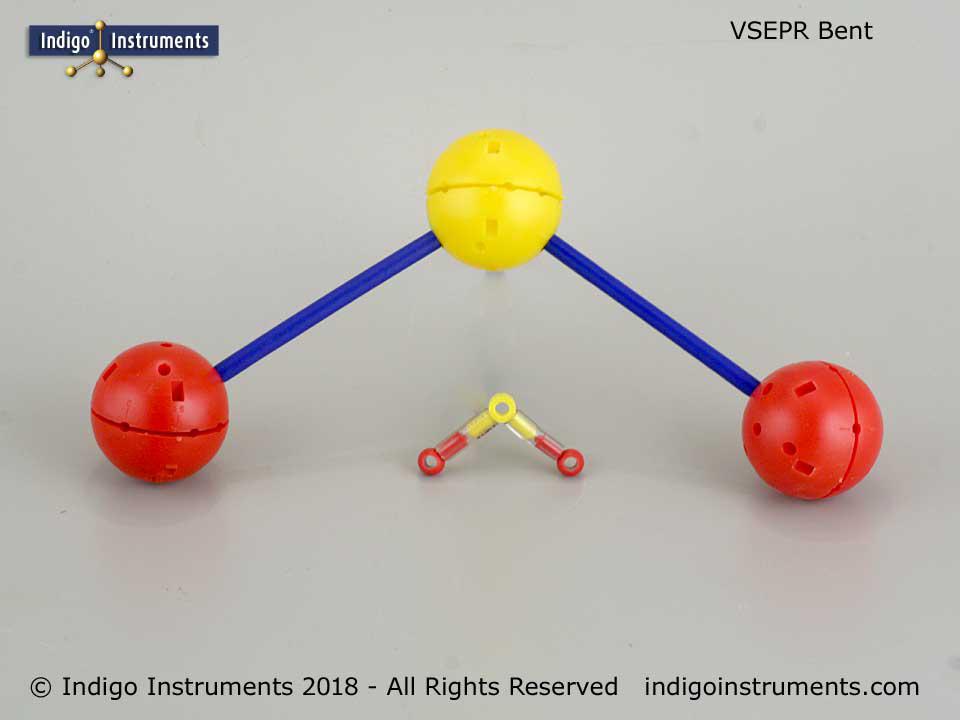
It is only possible when they are as far apart as possible. The electron pairs will rearrange themselves to minimize the repulsion.
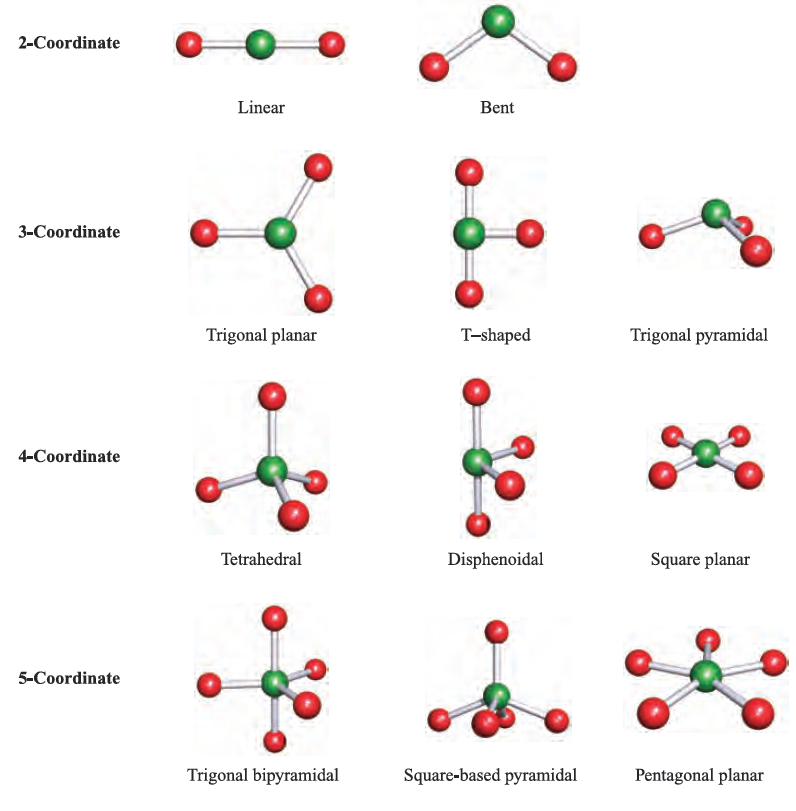
According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. The valence shell electron pair repulsion theory or VSEPR theory is used to predict the three-dimensional shape of a molecule. Therefore, we introduce another theory that reveals the molecular shape. However, the limitation of Lewis structure is that it gives no information about the arrangement of atoms in space. It also indicates where the lone electron pairs are located. Lewis structure is a straightforward way of representing the number and the type of bonds in a molecule.


 0 kommentar(er)
0 kommentar(er)
